CXA121 Histology Tutorial - Staining
1. Why is it important to have contrast between different cell components? Name some cellular components that can be distinguished using different stains.
By providing contrast between different cellular components we can demonstrate either normal cell morphology, or pathological changes. Colour contrast forms the basis of light microscopy. Histology utilises the cellular changes that can be demonstrated through staining. Commonly in the case of carcinoma we see the haematoxylin a basic dye bind to the nucleic acids. To contrast this eosin binds to the basic elements of the cytoplasm providing a counterstain. Virtually all cellular elements can be highlighted by different stains. For example:
- elastic fibres - Verhoeff EVG
- sulphated mucosubstance glycoproteins - Alcian blue PAS
- reticulin fibres - sliver retic method
Some images are shown at the end of the document.
2. Describe the general chemical composition of dyes with reference to the dye, chromogen and auxochrome.
A dye is a molecule, often an aromatic ring structure with a carbon backbone. Bound to this is one or more chromogen. A chromophore is the part of a molecule responsible for its colour. This region in the molecule is where the energy difference between two different molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can thus be absorbed by exciting an electron from its ground state into an excited state. In the conjugated chromophores, the electrons jump between energy levels that are extended pi orbitals, created by a series of alternating single and double bonds, often in aromatic systems. An auxochrome is a functional group of atoms with nonbonded electrons which cannot provide colour but when attached to a chromophore, alters both the wavelength and intensity of absorption. An auxochrome helps a dye to bind to the object that is to be coloured. Electrolytic dissociation of the auxochrome group helps in binding and it is due to this reason a basic substance takes an acidic dye.
3. Briefly describe how a chromogen can infer colour with reference to the 'aromatic' ring structure and delocalised electrons.
Unlike most organic compounds, dyes possess colour because they 1) absorb light in the visible spectrum (400-700 nm), 2) have at least one chromophore (colour-bearing group), 3) have a conjugated system, i.e. a structure with alternating double and single bonds commonly an 'aromatic' ring, and 4) exhibit delocalised electrons, which is a stabilizing force in organic compounds. Visible light that hits the chromophore can thus be absorbed by exciting an electron from its ground state into an excited state.
4. Explain what is meant by basic/acidic dyes.
This does not denote the pH of the dye solution. Ionic bonding involves electrostatic attraction between oppositely charged ions. One ion is a fixed ion in the tissue section and the other is the dye ion. Anionic (negatively charged or acidic) dyes will bind to cations (positively charged) in the tissue, and cationic dyes or basic will bind to tissue anions. Ionic bonding is the single most important form of bonding in most histological staining. Almost all simple staining can be understood and controlled by understanding the ionic charges involved.
- Haematoxylin is a basic dye and binds to acid
- Eosin is an acidic dye and binds to basic components
5. Outline the different factors that can affect staining.
Orthochromasia: tissues stained by either one dye or the other e.g. pink (eosin), blue (haematoxylin)
Polychromasia: tissues stained by a combination of both dyes 'layering' effect e.g. Romanowsky stains that use methylene blue and eosin Y. The methylene blue is demethylated and produces a range of dyes, and combined with eosin stain different parts of the cells a range of different colours that can't be attributed to the methylene blue or the eosin alone.
Metachromasia: tissue stains a different colour to the dye 'colour shift' e.g. Toluidine blue stains mast cells - specifically the granules in Mast cells (tissue cells similar to basophils) a red/purple colour and the rest of the tissue stains blue
Concentration of the Dye: the greater the concentration of the dye, the more the dye is bound to tissue components.
Temperature: an increase in temperature increases the rate at which the dye diffuses throughout the tissue sample. It can also alter tissue components so that they are more receptive to dye penetration.
pH of the Staining Solution: cells and other tissue elements often have an affinity for stains/dyes with specific pH ranges. Thus, the pH of the staining solution can have a direct impact on the ability of a dye to bind with its intended tissue element. Staining depends largely on the attachment of dyes to proteins. These have both positively and negatively charged groups. In proteins we are concerned with positively charged amino groups, and negatively charged carboxyl and hydroxyl groups. In addition, phophate groups of DNA are important in nuclear staining. Dyes also have the same groups as the proteins, but may include the sulphonic group as well. Which of these groups is involved in any particular case depends on the circumstances, including the pH of the staining solution.
Tissue fixation: fixation alters and reorganizes certain molecular structures in tissue samples so that they have an increased permeability and are more receptive to staining. Unfixed tissue elements have limited binding sites for dyes.
6. Explain the role of a mordant in dye binding
Mordanting is the use of a non-dyeing compound to improve the binding of the dye, with the mordant involved being able to mediate a dye-tissue interaction. The mordant (often a metal salt) acts as a link between the dye and the tissue. The mechanism by which the mordant binds to the tissue is not certain but one likely mechanism is covalence bonding resulting in a chelate that is stable. The dye and mordant complex is sometimes called a dye lake.
Since it is the mordant that binds to the tissue, the selectivity of the dye is controlled by selecting the mordant not the dye. The mordant gives greater stability to the stain and is not easily removed by water, alcohols or weak acids (i.e. it is a fast dye) and this makes it ideal when other stains are to be used afterwards, as the stain resists decolourization by the later reagents.
7. Describe the differences between progressive and regressive H&E staining mechanisms. List some pros and cons for each method.
Progressive: hematoxylin primarily stains colour chromatin and to a much less extent cytoplasm to the desired optical density, regardless of the length of staining time. Regressive haematoxylin over stain the chromatin and cytoplasm and requires subsequent immersion in dilute acid alcohol to pull out the excess colour from the chromatin and cytoplasm. If differentiation is omitted or incomplete, residual haematoxylin visually obscures fine chromatin detail and can prevent the uptake of eosin.
Progressive solutions stain to a desired intensity and no more. Therefore they do not require differentiation in a dilute acid alcohol. Commonly, for solutions such as Mayer's or Gills, a staining time of 5-10 minutes is used. Progressive staining solutions are fairly tolerant of minor variations in the application time, and a few minutes will not usually make much of a difference once a significant degree of staining has taken place. Progressive solutions initially tend to stain rapidly to a point determined by the relative amounts of dye and mordant, then to self-limit further staining. This is not an absolute, as staining does intensify, but it does so at a much slower rate than the initial coloration. Regressive staining means that the tissue is deliberately over stained and then de-stained (differentiated) until the proper endpoint is reached. The difference between methods is largely one of convenience. Progressive haematoxylins are generally less concentrated and work slowly to avoid overshooting the endpoint. Regressive haematoxylins are more concentrated and many can achieve over staining in a matter of less than a minute, while differentiation (removal of excess stain) requires only a few seconds. Also, timing is not so important in regressive procedures. As long as the slide is over stained, it doesn't matter by how much (within reason).
Progressive: doesn't require differentiation, slower staining process, easier for smaller labs, generally slightly longer overall time.
Regressive: requires the addition of a differentiation step, more rapid staining (good for frozen sections) can be easily automated as variation in haematoxylin batches are compensated for by over staining, sensitive to tap water pH swings.
8. Explain the difference between H&E 'blueing' and acid alcohol differentiation.
Differentiation and bluing (blueing, if you prefer English over US spelling) are essential to satisfactory staining by haematoxylins. Differentiation is used only with regressive haematoxylin formulations, while bluing is used with both regressive and progressive haematoxylin formulations. Differentiation effects quantitative changes; bluing, qualitative. Differentiation can be accomplished in several different ways, the usual method for hemalum is acid ethanol. This is usually 0.5% - 1% hydrochloric acid in 70% ethanol (standard acid alcohol). The purpose is to remove any haematoxylin from the tissue that is not bound by the mordant. Blueing: When applied from an acid medium, as most hemalums are, the initial colour of the material stained brick red. This is not permanent and must be changed to blue. The red phase of alum-hematein is soluble, and will slowly leach from the structures into whatever medium is used under the coverslip. The blue phase is insoluble and is generally considered to be permanent. The change is made by altering the pH. It is customary to place the stained and differentiated sections into an alkaline medium for a few minutes to convert the stain, then to wash with water to remove whatever was used to alter the pH.
Point of Interest: Following blueing in a dilute alkaline solution the sections must be washed to remove excess blueing agent. When this is done it is important to ensure that the wash water itself is not acidic and does not reconvert the blue hemalum back to red, even partially. Distilled water is usually satisfactory but, if not, a very small amount of an alkalising agent may need to be added. The pH should be just on the alkaline side of neutrality, but if the wash water is too alkaline then subsequent staining with eosin can be inhibited.
9. Explain the role of graded alcohol and xylene in the H&E staining protocol.
The graded alcohols function as an intermediate to take the section either from xylene to water or vice versa. This is required as xylene is not miscible with water and is required for the dewaxing (first) and mounting (last) steps of staining. Xylene is used to remove wax from the sections allowing aqueous staining solutions to penetrate the tissue. It is also used as the solvent is most mounting mediums. These are the glue like substances that will permanently fix the coverslip to the section.
Please read step 52 - 61 of '101 Steps to Better Histology' under the Prac Resources tab on MyLO and work your way through the troubleshooting of the stains.
Bronchial biopsies from a smoking lung
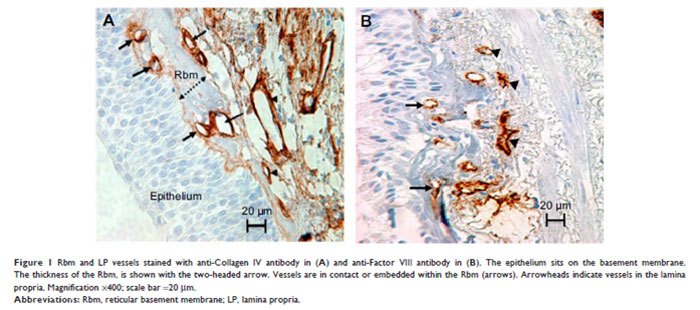
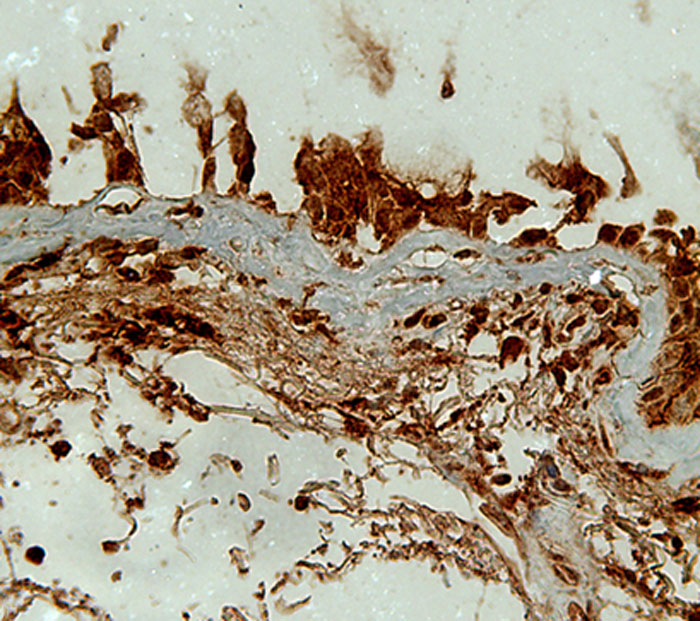
Figure 2: Bronchial biopsy from a smoking lung stained for a proteolytic enzyme - matrix metalloproteinase-9 (MMP-9; Brown colour)
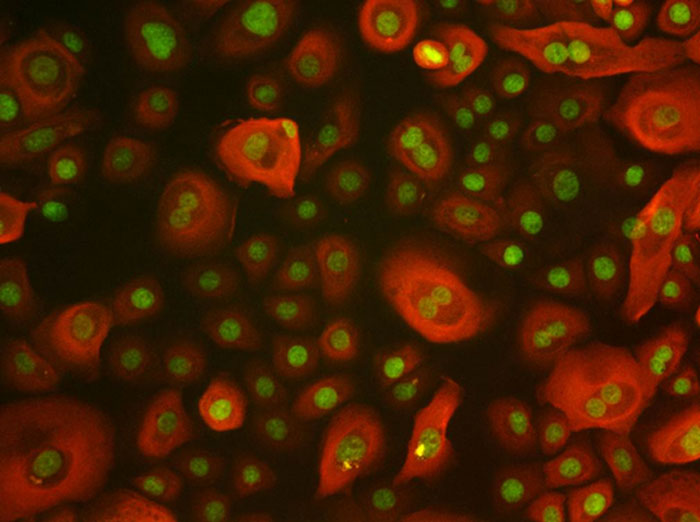
Figure 3: Epithelial cells stained for cytokeratin (red) and green is nuclear stain.
Except where otherwise noted, content on this page is licensed under a Creative Commons Attribution-ShareAlike 4.0 International Licence
Note: The open version of this document may have been altered from the original. Only pages on this site that display the CC licence and logo are licensed under a Creative Commons licence.
| Attribution information | |
|---|---|
| Title: | CXA 121 - Histology - Tutorial - Staining |
| Source: | https://www.utas.edu.au/health/resources/open-resources/resources/courses/laboratory-medicine/cxa-121-histology-tutorial |
| Author: | Sukhwinder Sohal |